
- Inspiring People -
- 4mins -
- 32 views
Test to detect novel coronavirus in as little as five minutes receives FDA’s go-ahead
Medical devices and healthcare company Abbott announced it has received emergency FDA authorisation for a molecular point-of-care test test that is capable of delivering positive results of the coronavirus in as little as five minutes.
ABBOTT LAUNCHES MOLECULAR POINT-OF-CARE TEST TO DETECT NOVEL CORONAVIRUS IN AS LITTLE AS 5 MINUTES
American medical devices and healthcare company Abbott on Friday announced it has received emergency use authorisation (EUA) from the US Food and Drug Administration (FDA) for the fastest available molecular point-of-care test for the detection of novel coronavirus (COVID-19), delivering positive results in as little as five minutes and negative results in 13 minutes.
Abbott says it plans to ramp up production to deliver 50,000 ID NOW COVID-19 tests per day, beginning next week, to the US healthcare system. This is the company’s second test to receive Emergency Use Authorisation by the FDA for COVID-19 detection; combined, Abbott expects to produce about 5 million tests per month.

Portable test machine can be used in more non-traditional places not only hospitals
What makes this test so different is where it can be used: outside the four walls of a traditional hospital, in places such as in the physicians’ office or urgent care clinics.
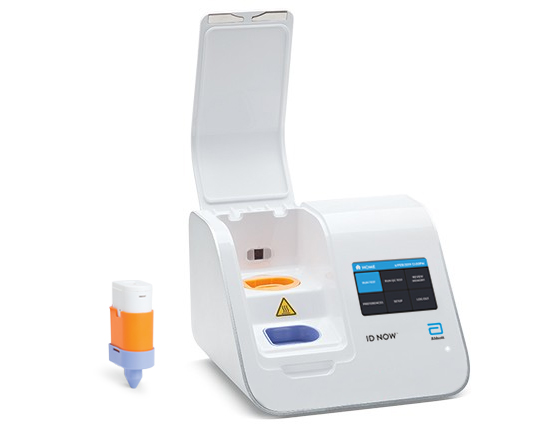
The new Abbott ID NOW COVID-19 test runs on Abbott’s ID NOWTM platform—a lightweight box (6.6lbs/3kg and the size of a small toaster) that can sit in a variety of locations. Because of its small size, it can be used in more non-traditional places where people can have their results in a matter of minutes, bringing an alternate testing technology to combat the novel coronavirus.
“We’re ramping up production to deliver 50,000 ID NOW COVID-19 tests per day, beginning next week, to the U.S. healthcare system.” — says Abbott on their website.
The news comes on the heels of Abbott’s announcement last week of the availability of their Abbott RealTime SARS-CoV-2 EUA test under FDA EUA, runs on m2000 RealTime molecular system for centralised lab environments. Learn more
Combined with ID NOW, Abbott expects to produce about 5 million tests in April.
Testing remains a crucial step in controlling the novel COVID-19 pandemic. Continuing to supply healthcare providers with new technologies to help curb the spread of infection is a top priority for public health officials.
Source: Abbott
Why taking molecular testing to the front lines Is a game-changer
Molecular point-of-care testing for COVID-19 offers healthcare workers rapid results in more settings where people show up for care. Molecular testing technologies help detect the presence of a virus by identifying a small section of the virus’ genome, then amplifying that portion until there’s enough for detection.
This process can cut testing wait time from hours, if not days, to as little as five minutes for positive results and 13 minutes for negative results.
When not being used for COVID-19 testing, ID NOW is the leading molecular point-of-care platform for Influenza A&B, Strep A and respiratory syncytial virus (RSV) testing. The platform holds the largest molecular point-of-care footprint in the US and is already widely available in physicians’ offices, urgent care clinics, and hospital emergency departments across the country.
"Through the incredible work of teams across Abbott, we expect to deliver 50,000 COVID-19 tests per day to healthcare professionals on the front lines, where testing capabilities are needed most," said Chris Scoggins, senior vice president, Rapid Diagnostics, Abbott. "Portable molecular testing expands the country’s capacity to get people answers faster."
Please note: The ID NOW COVID-19 EUA has not been FDA cleared or approved. It has been authorised by the FDA under an emergency use authorisation for use by authorised laboratories and patient care settings. The test has been authorised only for the detection of nucleic acid from SARS-CoV-2, not for any other viruses or pathogens, and is only authorised for the duration of the declaration that circumstances exist justifying the authorisation of emergency use of in vitro diagnostic tests for detection and/or diagnosis of COVID-19 under Section 564(b)(1) of the Act, 21 U.S.C. § 360bbb-3(b)(1), unless the authorisation is terminated or revoked sooner.
Source: Abbott

